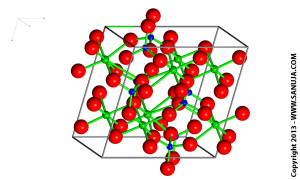
Click on the image for larger high resolution file.
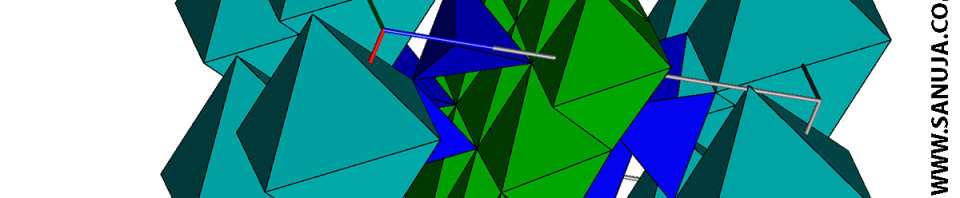
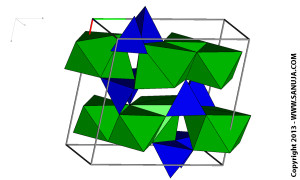
Dark blue is Silicate Tetrahedra, green is bonds and the light blue is sodium.
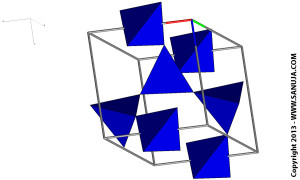
The isolated tetrahedra is a group of silicates in which the crystal structures have no shared oxygen between them. Therefore minerals like Olivine and Garnet are held together by the attaraction force between silica tetrahedra and the surrounding cat ions. This is the least polymerized type.
Single chain silicates have links between the silicate tetrahedras by sharing two oxygen atoms between two of them. Pyroxene is the most common group in this category.
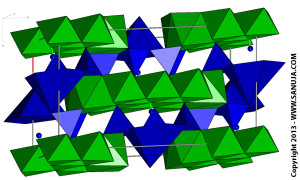
Double chain silicates shares two or three oxygen atoms. You can look at these structures as two independent chain silicates bonding together because the fundamentally, they are two set of chains. Amphiole group is an example of such minerals.
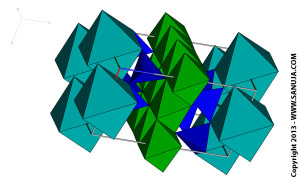
Sheet silicates form 2D silicate structures. All sheet silicates share exactly three oxygen atoms between silica tetradera. While sheet themselves are strong, in between the sheets different types of molecules can be fitted in. One for the example of this would be the water molecule in which a hydrous form of the mineral is formed. Micas such as biotite and muscovite are examples of such minerals.
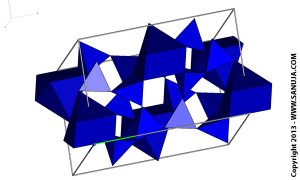
Framework silicates are the most polymerized version. The structure uses all four oxygen atoms in the silica tetrahedra to form bonds between them. Feldspars are the most common type of framework silicates.