Go to: Final Exam
Notice
Most of the quiz updates are completed. If you find any errors, please let me know.Mineralogy (GLGY 311-UCAL) Midterm Exam
Congratulations - you have completed Mineralogy (GLGY 311-UCAL) Midterm Exam.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Credits: Based on the excellent class notes provided by, Mr. William Matthews
Some of the lab samples and mineral data | Click here
Notice
10 Multiple Choice questions very similar to what will be on the Midterm and the Final Exam. You should be able to do it in 10 min or less!Mineralogy (GLGY 311-UCAL) Silicates Quiz
Congratulations - you have completed Mineralogy (GLGY 311-UCAL) Silicates Quiz.
You scored %%SCORE%% out of %%TOTAL%%. With incorrect multiple attempts your score is %%PERCENTAGE%%
Your performance has been rated as %%RATING%%
Basic Mineralogy Concepts
Mineral Identification
Mineral Identification chart with few minerals for first and second year students. If you would like to download the PDF file, please right click here and save it on your computer.
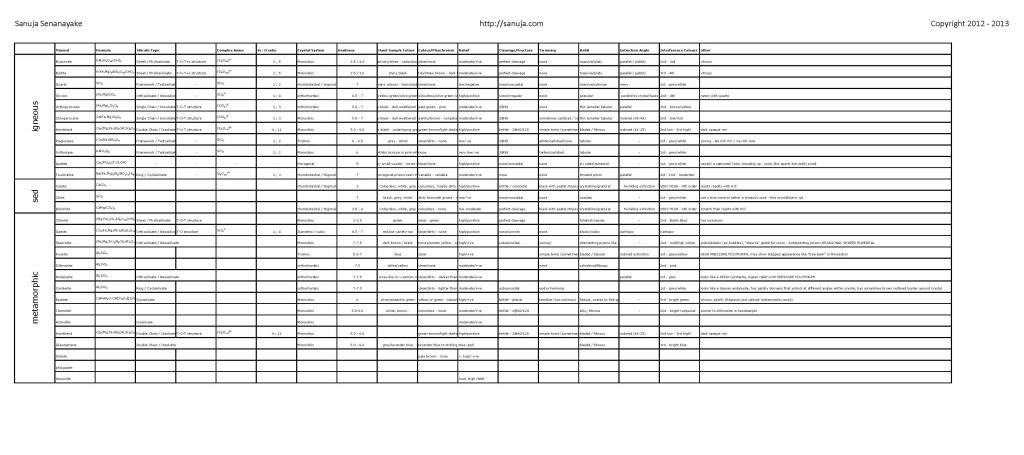
If you don’t know how these descriptions are derived, please read the textbook, lecture notes or info posted below.
Mineral Properties
Check out these great videos of minerals under PPL and XPL at: YouTube. We need more geologists like this in our schools!
- cleavage: in mineralogy it is a plane of weakness in the mineral structure along which it can break.
- colour: while colour under petrographic microscope is a good useful properties, it is not as good when using hand samples. Sometimes the colour in hand sample such as salmon pink colour in hand sample may be used as a diagnostic property of k-feldspar.
- crystal form: the crystal’s physical structure give it’s shape. It is a diagnostic property which sometimes described as crystal habit.
- fracture: info here
- hardness: it is as simple as soft vs hard.
- luster: info here
- magnetism: iron rich minerals and rocks can be identified using this property.
- HCl test: calcium carbonate minerals and other carbonate based minerals will from bubbles.
- specific gravity: not usually used in the field but rather by companies and research groups in a laboratory environment.
- streak: unlike colour of a rock, streak colour is the colour of the powder of the rock.
- taste: limestone and calcite will taste like salt. Unless otherwise you must taste something, DO NOT put anything in your mouth.
Mineral Hardness(Mohs) Scale
| Hardness | Mineral | Household Item |
| 1 | Talc | ? |
| 2 | Gypsum | Salt (2.3) Finger nail (2.5) |
| 3 | Calcite | Gold (2.5-3) |
| 4 | Fluorite | Household Item |
| 5 | Apatite | Copper coin (5) Glass window (5.5) |
| 6 | Feldspar | Pen knife (6.5) |
| 7 | Quartz | ? |
| 8 | Topaz | ? |
| 9 | Corundum | ? |
| 10 | Diamond | ? |
| > 10 | Diamond nanorods | ? |
[To Top]
Mineral Structure
Several ions in a particular arrangement is the basic building blocks of all minerals known as motif. To create structures out of these blocks, several motifs are connected to each other creating a lattice. Lattice is like a plane of motifs and therefore, but connecting several lattice sheets in a 3D settings, we get a unit cell. Since the unit cells are 3D, they have the ability to arrange together in different configurations. These different configurations of unit cells creates the crystal system. The crystal system defines the shape of crystals. There are many different types of crystal systems. But one of the most common type on Earth is silicates, which we will discuss in detail.
Silicate Classes
- Orthosilicates = Nesosilicates: (SiO4)
- Disilicates = Sorosilicates: (Si2O76-)
- Ring silicates = Cyclosilicates: (SiO4 rings)
- Chain silicates = Inosilicates: (SiO32- or Si4O116-)
- Sheet silicates = Phyllosilicates: (Si2O5 and Si4O10-4)
- Framework silicates = Tectosilicate: (SiO2)
– Si to O ratio of 1:4
– least degree of polymerization
– no oxygen anions are shared between adjacent tetrahedra
– have no connections between Si to Si atoms, but connected by cations
– Si to O ratio of 2:7
– share a single O2- between two silicon tetrahedra
– only a small group of minerals found in this form
– complex anion is Si6O18-12
– Si to O ratio of 1:3
– many different configurations.
– share two O2- per tetrahedra and forms rings
– usually, not always six sided
– only a small group of minerals found in this form
– rings froms columns by stacking on each other
– adjacent columns are linked by cations and the center of the columns may be filled with cat/an ions
– Si to O ratio of 4:11
– two types; a) singale chains with SiO32- and b) double chain with Si4O116-
– TOT; tetraheral – octahedral – tetrahedral
– Si to O ratio of 2:5
– share three O2- per tetrahedra to form continuous sheets
– most abundant and important minerals found in this class
– all levels of rocks such as igneous, metamorphic and sedimentery
– Si to O ratio of 1:2
– the most polymerized silicate class
– very importnat because more than half of minerals in Earth’s crust has this feature
Crystal Systems
Please refer to the diagram click here.
Isometric
a1 = a2 = a3 = 90o
α = β = γ = 90o
Tetrgonal
a1 = a2 ≠ c
α = β = γ = 90o
Hexagonal
a1 = a2 = a3 ≠ c
Orthorhombic
a ≠ b ≠ c
α = β = γ = 90o
Monoclinic
a ≠ b ≠ c
α = γ = 90o ≠ β
Triclinic
a ≠ b ≠ c
α ≠ β ≠ γ ≠ 90o
Ref: Northern Arizona Meteorite Laboratory